Beautiful Mdsap Audit Report
2 ISO 134852016 Standard Checklist.
Mdsap audit report. Ad Find Mdsap Audit. Application of MDSAP Audit Reports. 4 Essential Principles Checklist TGA.
1 MDSAP - ISO 13485 Audit Checklist. Ad Find Mdsap Audit. 3 MDSAP Audit Checklist FDA.
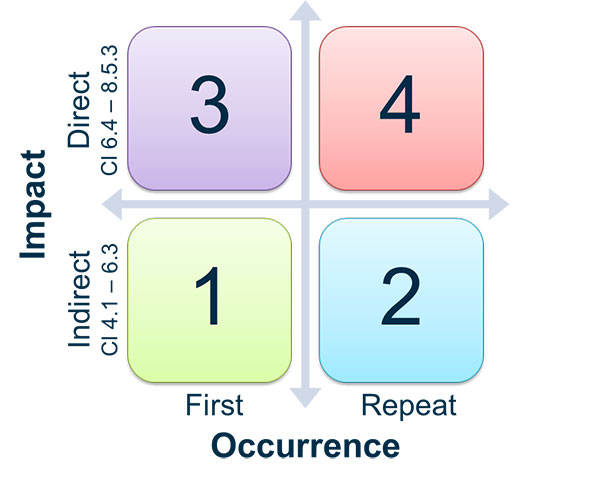
Can this be a problem to get certified. But this depends on the grading of the observation. Ad Find Mdsap Audit.
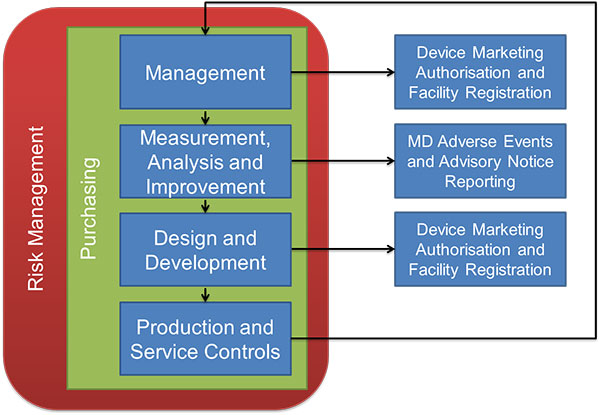
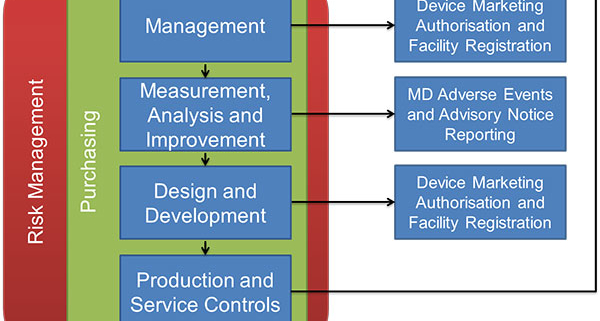
Ad Find Mdsap Audit. But if during the audit some issues were raised this can lead to some observations. Your MDSAP audit reports will be accessible via a central repository to the five participating regulators in the US Canada Australia Brazil and Japan but they will not be.
Open the document in our online editing tool. This consultation is now closed. MDSAP Medical Device Regulatory Audit Reports.
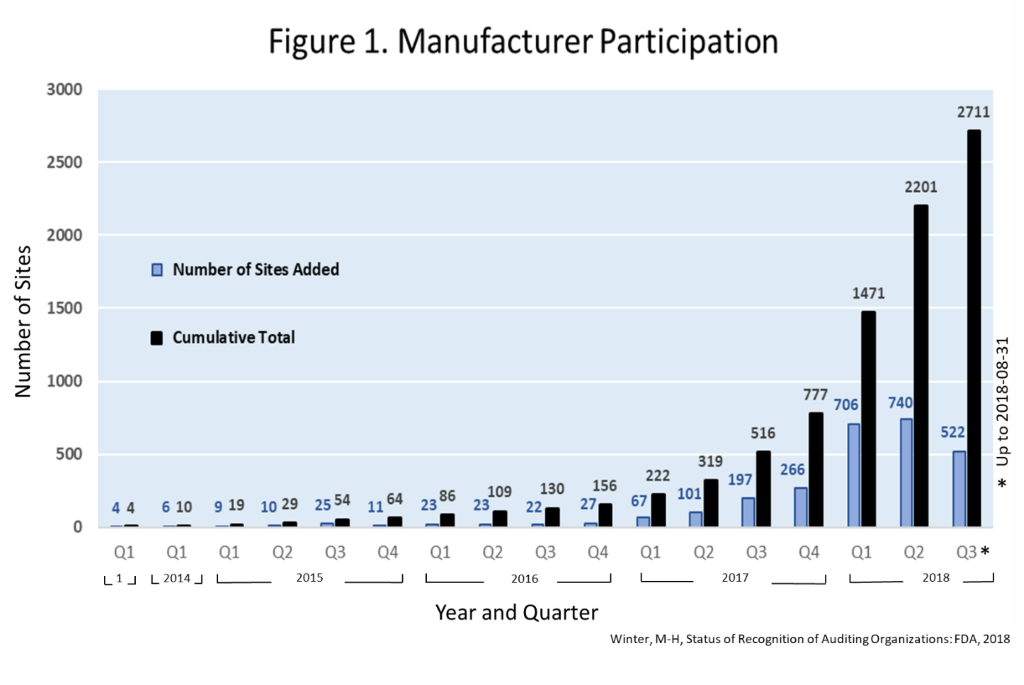
On 29 June 2017 a report was generated summarizing the outcomes of prospective proof-of-concept criteria established to confirm the viability of the Medical Device Single Audit Program. Find gaps in your QMS and maintain the quality of medical devices. Read the instructions to determine which details you will need to provide.
/tuv-rheinland_mdsap-visual-en-update_core_1_x.png)